FREE STANDARD OPERATING PROCEDURE (SOP) EXAMPLES & RESOURCES
ARE YOU LOOKING FOR HIGH QUALITY STANDARD OPERATING PROCEDURE (SOP) EXAMPLES FOR YOUR COMPANY?
Find out how our SOP Software helps companies improve their operations. |
ADVANTAGES AND BENEFITS:
WE HAVE OVER 30 YEARS EXPERIENCE IN HELPING COMPANIES IN A VARIETY OF INDUSTRIES CREATE WORK INSTRUCTIONS, POLICIES, PROTOCOLS AND SOPS.
- Use other standards as examples
- improve operations & performance
- reduce risk
- lower cost
- maintain quality and safety
- adhere to regulations
- avoid costly disruption
WHAT YOU WILL GET:
- For a variety of examples, select the template that best fits for procedures, processes, and protocols included in our software
- Automatically generate your SOP training and job aids in minutes
- Deploy your SOPs anywhere.
STANDARD OPERATING PROCEDURE SOFTWARE SYSTEM:
SOP EXPRESS
Create SOPs, Generate Work Instructions, Visual Job Aids, Quick Guides & Checklists
EXPRESS TRAIN
Generate Training Materials, Web-based Tutorials, Online Quizzes, PowerPoint Slides, & Guides
SOP TRACKS
Connect to SharePoint. Track SOP Versioning, Document History, Workflow for Approval, Single Source Store
SCORM LMS PACKAGER
Package Modules for Any SCORM Conformant Learning Management System to Record For Users, Groups, Regions, Courses, Assignments, And Mastery
WE CAN ALSO HELP YOU BEGIN THE PROCESS WITH EXAMPLES FROM OTHER CLIENTS:
- You can view examples of templates for created by other companies in your business marketplace.
- You can use examples of consistent standard operating procedures or work instructions
- You may review how other companies optimized their use of your existing assets and resources.
- We can help convert legacy content
- We can help you shape their process to fully leverage the benefits of our software
- We can show you how to use workflows in SharePoint to manage review and approval
- We can provide a front-end analysis of their requirements and processes to support your environment
HERE’S HOW IT WORKS
- Review and example of how to enter all your content into our Microsoft Word templates
- Select an example procedures such as equipment, product, process or protocol.
- See how other organizations define steps in each procedure
- Review how to identify the costly, dangerous and/or frequently used tasks.
- See how others have added images, graphics, tables, and even videos to help the operator better understand the task.
- Review how quizzes and knowledge checks can be used to verify understanding.
FORTUNE 500 AND SMALL COMPANIES ALIKE HAVE SEEN UP TO:

SAVINGS IN SOP DEVELOPMENT
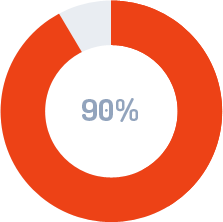
SAVINGS ON UPDATES AND REVISIONS
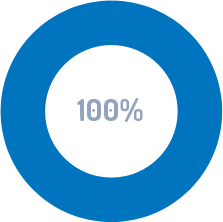
RETURN ON INVESTMENT WITHIN THE FIRST YEAR
When calculating the time and effort required developing SOPs along with the associated Job Aids and Training Materials.
SEE HOW OTHERS HAVE AUTOMATICALLY BUILT THEIR SOP TRAINING AND JOB AIDS IN MINUTES INCLUDING EXAMPLES OF:
- Work Instructions
- User Guides and user manuals,
- Checklists and Quick Reference Cards,
- Supervisor Task Qualification Checklists,
- Training Programs with PowerPoint presentations
- Web-based Training and Online Quizzes
SEE EXAMPLE OF HOW TO SYNCHRONIZED YOUR SOPD FROM ONE SOURCE!
- Enter your SOPs one time, and automatically generate all of your materials from one source!
- See how other companies keep their SOPs Consistent, Compliant and concurrent
See how easy it is to update and synchronize all your SOPs from one single-source.
WE AN SHARE OUR 30 YEARS OF EXPERIENCE HELPING COMPANIES ACHIEVE THEIR GOALS
- Pharmaceuticals Examples of capturing SOPs and generating checklist and job aids for clean-rooms
- Manufacturers Examples of creating work instructions for equipment set-up, operations, shut-down, and downtime maintenance protocols
- Hospitals Examples of capturing Lab Protocols with supporting checklists and quick reference cards
- Logistic companies Examples of training remote control crane operators at ports
- Government Agencies Examples of standardizing training and support across multiple international locations
- Aluminum plants Examples of standardizing SOPs and Work Instructions fully integrated with SharePoint at some of the largest international location
- Examples of Small and medium size companies utilizing our software as large companies do
STANDARD OPERATING PROCEDURE (SOP) SOFTWARE
OUR END-TO-END SOP SOFTWARE ENABLES YOUR ORGANIZATION TO:
- Create/convert your Standard Operating Procedures (SOPs) and automatically turn them into Visual Job Aids, Quick References, Training, PowerPoint, On-the-Job Checklists, Task Qualifications, Quizzes, and more.
- Track version and approval workflow.
- Track employee training results.
- Set Globally Unique Identifiers to ensure version consistency and audit-ability.
- Simplify your process of producing job aids and training materials for specific jobs, utilizing Standard Operating Procedure (SOP) Templates.
- Sync your content, with single edits rippling through outputs automatically.
FREQUENTLY ASKED QUESTIONS & ANSWERS
You can designate any location as a publishing location for the outputs from the SOP Express Desktop. This can be a SharePoint, a Shared Drive or any local/external drive. The outputs can be organized into easy-to-build indexes directly from the SOP Express Desktop and can then be shared with end users as links.
Yes, SOP Express includes all the functionality to generate your Reports (SOP, Polices, Work Instructions Assessments, and Supervisor Task Qualification Audits) as well your Job Aids (Checklists and Quick Reference Cards).
Yes, once we receive the order we will give you a list of items that will help us create the appropriate output templates for you. These usually include, your logo, your header/footer, any representative images (for backgrounds), etc.
The Maintenance fee includes all updates to the software, maintenance releases, and improvement releases for one year (renewable annually) … it also includes support for the first 90 days with access to our on-line ticket system for reporting issues or problems (which we monitor daily) … we can offer additional support and custom development services as needed. Just talk with us.
Actually the online self-paced training is made available for the first 90 days (plenty of time to complete) … and then online support materials are made available (job aids, quick reference cards, checklists, etc.) via a URL link with unlimited access.
If you do not have SharePoint, then an outline index can be created and moved to any HTTP (URL) location. Your IT group may insert a sign-on and password for any user to get access to the URL (this is done on your end) … you can only have one sign-on and password for a give URL … so when we use this approach we establish a single sign-on and password for a group to access their index. With SOP Express you can set up as many outline view Indexes (with any number of modules) for as many groups as needed.
